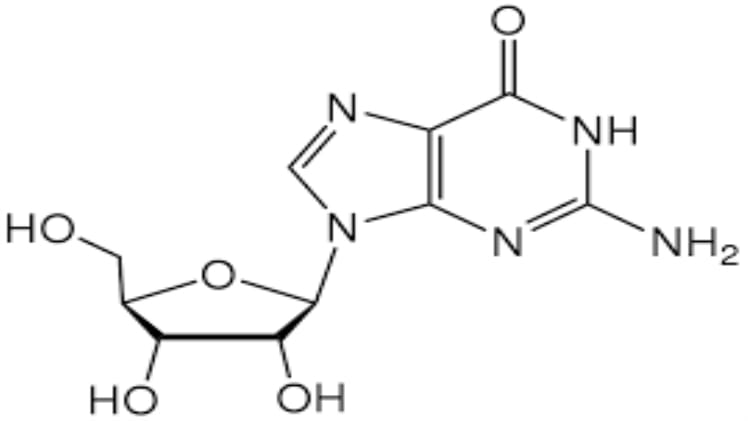
Guanine and guanosine are two closely related compounds found in nature. Both are nitrogenous bases, which are important components of nucleic acids. While guanine and guanosine are very similar in structure, there are some subtle differences between the two that can be important to consider when studying their properties.
What is Guanine?
Guanine is a purine base found in DNA and RNA. It is one of the four main nitrogenous bases that form the building blocks of nucleic acids. Guanine is a white crystalline compound with a melting point of around 180°C. It is soluble in water and alcohol, and insoluble in non-polar solvents. Guanine has a structure similar to adenine, and is composed of a planar six-membered ring with five carbon atoms and one nitrogen atom. It is an aromatic compound, meaning that it can form resonance structures.
What is Guanosine?
Guanosine is a nucleoside composed of guanine and the sugar ribose. It is found in both DNA and RNA, and is one of the four main nitrogenous bases that form the building blocks of nucleic acids. Guanosine is also a white crystalline compound with a melting point of around 180°C. Unlike guanine, guanosine is soluble in both polar and non-polar solvents. It is composed of a single six-membered ring with five carbon atoms and one nitrogen atom, in addition to the sugar ribose. Guanosine also forms resonance structures, and is an aromatic compound.
In conclusion, guanine and guanosine are very similar compounds, both of which are important components of nucleic acids. While their structures are almost identical, there are some subtle differences between the two, including their solubility and the presence of the sugar ribose in guanosine. It is important to consider these differences when studying their properties.